Understanding the Inflammatory Cascade
Autoimmune arthritis, a complex and debilitating condition, is characterized by chronic inflammation in the joints. This inflammation is driven by a complex cascade of events, involving a multitude of immune cells and signaling molecules. Understanding the intricate details of this cascade is crucial for developing effective therapeutic strategies. The cascade begins with the recognition of self-antigens by the immune system, a process often triggered by environmental factors or genetic predispositions. This recognition leads to the activation of immune cells, which then release inflammatory mediators, further amplifying the inflammatory response.
This cascade of events is a tightly regulated process, but when dysregulation occurs, it can result in prolonged and damaging inflammation. The inflammatory response involves a coordinated effort from various components of the immune system, including macrophages, neutrophils, and lymphocytes. These cells release cytokines, chemokines, and other signaling molecules, which further amplify the inflammatory response and contribute to the damage of joint tissues. The sustained release of these mediators leads to the erosion of cartilage and bone, the hallmark of autoimmune arthritis.
Gene Editing as a Potential Therapeutic Strategy
Given the complexity of the inflammatory cascade in autoimmune arthritis, traditional therapies often have limitations in effectively targeting the underlying mechanisms. Gene editing technologies, particularly CRISPR-Cas9, offer a promising new approach. These technologies allow for precise manipulation of genes involved in the inflammatory cascade, potentially offering a more targeted and effective treatment strategy. By directly targeting specific genes that contribute to the inflammatory cascade, gene editing could potentially disrupt the cycle of inflammation and reduce the destructive effects on the joints.
Specific gene targets for gene editing in autoimmune arthritis could include genes that encode inflammatory cytokines or chemokines. By editing these genes to reduce or eliminate their expression, gene editing could potentially dampen the inflammatory response. Beyond targeting inflammatory mediators, gene editing could also be used to restore the balance of the immune response, potentially by targeting genes involved in immune cell activation or regulation. This precise approach could minimize off-target effects and potentially offer a more sustainable solution compared to current treatments.
Challenges and Future Directions
Despite the promising potential of gene editing, significant challenges remain in translating this technology into effective therapies for autoimmune arthritis. One key challenge is the precise delivery of gene editing tools to the affected joints. Ensuring the specificity and efficiency of gene editing is crucial to avoid unintended consequences. Additionally, the long-term effects of gene editing on the immune system need further investigation. Long-term safety and efficacy studies are essential to ensure the safety and effectiveness of these therapies.
Future research should focus on developing more efficient and targeted delivery methods for gene editing tools to the joints. Further research is also needed to identify additional key genes involved in the inflammatory cascade and to develop more sophisticated gene editing strategies to target these genes. Understanding the complex interplay between genetic and environmental factors contributing to autoimmune arthritis is also crucial to optimize gene editing approaches and tailor them to individual patient needs.
Beyond CRISPR: Exploring Other Gene Editing Strategies
Beyond CRISPR: Gene Editing Beyond the Cas9 Enzyme
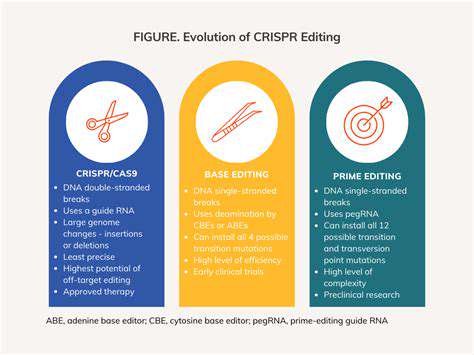
CRISPR-Cas9 has undeniably revolutionized gene editing, offering a powerful and precise tool for manipulating DNA. However, the limitations of CRISPR, such as off-target effects and delivery challenges, necessitate exploration of alternative gene editing methods. Scientists are actively investigating diverse approaches, broadening the possibilities for treating genetic diseases and advancing biomedical research.
These alternative methods are not simply incremental improvements; they represent entirely different strategies for achieving precise gene modifications. These approaches leverage various molecular mechanisms, ranging from programmable nucleases with different functionalities to base editors and prime editors. Each method offers a unique set of advantages and disadvantages, influencing their suitability for specific applications.
Exploring Base Editing and Prime Editing Technologies
Base editing, a relatively recent advancement, directly alters single DNA bases without the need for double-strand breaks. This minimizes the risk of off-target effects compared to CRISPR-Cas9, potentially leading to safer and more effective gene therapies. The ability to precisely modify DNA bases without inducing double-strand breaks is a significant step forward in the field.
Prime editing takes base editing a step further. It allows for more complex edits, including insertions, deletions, and even the rewriting of larger stretches of DNA. This versatility makes prime editing a promising tool for correcting complex genetic defects and potentially treating a wider range of genetic diseases. The potential to introduce more substantial modifications opens new avenues for tackling genetic disorders that were previously considered intractable.
The development and refinement of these technologies are ongoing, with researchers constantly working to improve their efficiency, specificity, and delivery methods. This continuous improvement is crucial for their widespread application in various biomedical fields.
Investigating Other Nucleases and Non-Nuclease Approaches
Beyond base and prime editing, scientists are also exploring other nucleases with distinct properties. These include engineered nucleases with improved targeting accuracy and reduced off-target effects. These advancements hold the promise of a more precise and reliable gene editing toolset.
Furthermore, non-nuclease approaches, such as transcription activator-like effector nucleases (TALENs) and zinc finger nucleases (ZFNs), have also been investigated. These methods, while initially promising, have not achieved the same level of widespread adoption as CRISPR-Cas9. However, ongoing research may uncover novel applications for these older technologies.
The overarching goal of this research is to develop versatile and reliable gene editing tools that can be used to address a wider range of genetic diseases and advance fundamental biological research. This exploration into diverse gene editing strategies will likely lead to the development of new therapies and diagnostic tools.
The quest for hypersonic flight, capable of speeds exceeding Mach 5, promises revolutionary advancements in global transportation. This technology holds the potential to drastically reduce travel times between continents, opening up new possibilities for international commerce and tourism. Imagine a world where transatlantic flights are measured in hours rather than days. This paradigm shift in air travel necessitates significant advancements in materials science, propulsion systems, and aircraft design to withstand the extreme temperatures and pressures encountered at such high speeds.

The Future of Gene Editing in Autoimmune Arthritis Treatment
Harnessing CRISPR for Precision Targeting
CRISPR-Cas9 gene editing technology offers a revolutionary approach to autoimmune arthritis treatment. This powerful tool allows scientists to precisely target and modify genes implicated in the disease process. By disabling or correcting faulty genes involved in immune system dysregulation, CRISPR-Cas9 holds the potential to significantly reduce inflammation and pain associated with autoimmune arthritis, potentially even leading to long-term remission.
The ability to precisely target specific genes related to immune cell activation and inflammatory pathways allows for a highly personalized approach to treatment. This precision is a crucial advancement over traditional therapies that often have broad effects on the immune system, potentially leading to unwanted side effects. Scientists are actively working on optimizing CRISPR delivery methods and minimizing off-target effects to ensure its safety and efficacy.
Personalized Medicine Approaches
The future of autoimmune arthritis treatment is increasingly moving towards personalized medicine, and gene editing plays a pivotal role. Understanding the unique genetic predispositions and specific immune responses of each patient is key to developing tailored therapies. By analyzing individual genetic profiles, researchers aim to identify the specific genes driving the autoimmune response in each patient, enabling the development of personalized gene editing strategies.
This approach could lead to more effective treatments with fewer side effects compared to current, one-size-fits-all strategies. The ability to tailor gene editing to individual needs promises a significant leap forward in managing this complex and often debilitating condition.
Beyond CRISPR: Exploring Other Gene Editing Tools
While CRISPR-Cas9 is currently at the forefront of gene editing research, other promising tools are emerging. These alternative methods, such as base editing and prime editing, offer the potential for even greater precision and efficiency in modifying specific DNA sequences. These newer techniques may overcome some limitations associated with CRISPR, such as off-target effects and limitations in modifying larger segments of DNA.
Addressing Ethical Considerations
The potential of gene editing for autoimmune arthritis treatment raises crucial ethical considerations. Discussions around the responsible development and implementation of these technologies are essential. This includes navigating the ethical implications of altering the human genome, ensuring equitable access to these potentially transformative therapies, and addressing potential long-term consequences.
Open dialogue and robust ethical frameworks are vital to ensure that gene editing technologies are used responsibly and benefit patients without compromising fundamental ethical principles.
Improving Delivery Mechanisms and Reducing Off-Target Effects
Delivering gene editing tools to the specific cells and tissues affected by autoimmune arthritis remains a significant challenge. Researchers are actively working on developing more efficient and targeted delivery methods to ensure that the gene editing agents reach their intended targets effectively. This includes exploring different delivery vectors and optimizing the timing and location of administration.
Minimizing off-target effects is another critical aspect of gene editing development. Improving the precision of gene editing tools and developing strategies to monitor and mitigate off-target modifications are crucial for ensuring the safety of these therapies. These advancements are essential for maximizing the therapeutic potential of gene editing while minimizing potential risks.
The Long-Term Potential and Future Research Directions
The long-term potential of gene editing for autoimmune arthritis treatment is immense. Successful clinical trials and continued research are crucial to translating this promising technology into readily available therapies. Further research will focus on understanding the long-term effects of gene editing, optimizing treatment protocols, and expanding the applications of gene editing to other autoimmune diseases.
Future research directions include investigating the potential of combining gene editing with other therapies, exploring the role of the microbiome in autoimmune arthritis, and developing diagnostic tools to identify patients who would benefit most from gene editing interventions. These advancements will pave the way for a new era of precision medicine in the fight against autoimmune arthritis.