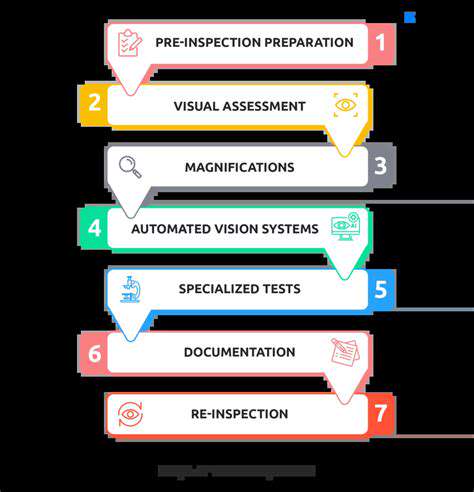
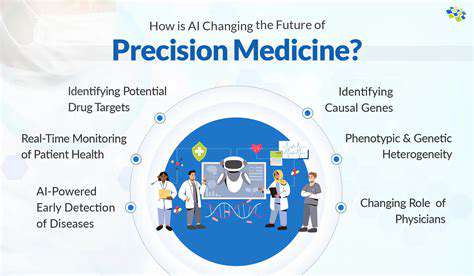
Overcoming Challenges in Translation
Bridging the Gap Between Bench and Bedside
Translation of research findings from the laboratory (bench) to clinical application (bedside) is a critical but often challenging process. This intricate journey necessitates meticulous attention to detail, a thorough understanding of the research methodology, and a strong grasp of the clinical context. Successful translation often hinges on effectively communicating the research's implications and potential applications to healthcare professionals and patients, ensuring the research's relevance and feasibility in real-world settings.
Many breakthroughs in biomedical research often languish in the laboratory, never making it to the clinic. This crucial step is not just about applying the findings; it's about adapting them to the needs of patients and the complexities of real-world healthcare, which requires careful consideration of ethical implications, regulatory hurdles, and practical implementation.
Translating Research Findings into Clinical Practice
A key aspect of overcoming challenges in translation involves effectively transferring research findings into clinical practice. This transition often requires adapting laboratory-based studies to the complexities of real-world clinical settings. Successful translation also demands the ability to communicate research findings in a manner that is accessible and understandable to healthcare professionals, ensuring that the information is not only accurate but also actionable.
Addressing Ethical Considerations in Translation
Translating research invariably involves ethical considerations that must be carefully addressed throughout the process. Ensuring the safety and well-being of patients is paramount, and the research must comply with ethical guidelines and regulations. This encompasses obtaining informed consent, protecting patient confidentiality, and navigating potential conflicts of interest.
Ethical review boards play a crucial role in ensuring that research translations adhere to ethical standards. Transparency and accountability are essential components of this process to build trust and confidence in the translated research and its subsequent applications.
Navigating Regulatory Hurdles in Translation
Translation often faces significant regulatory hurdles that need careful navigation. Different countries and jurisdictions have varying regulations regarding the approval and implementation of new treatments and technologies. Obtaining the necessary approvals and clearances can be a time-consuming and complex process, requiring expertise in regulatory affairs and a deep understanding of the legal frameworks governing healthcare.
Understanding the nuances of regulatory processes is critical for successful translation, ensuring that the translated research is compliant with all applicable laws and standards.
Developing Effective Communication Strategies
Effective communication is essential for translating research from bench to bedside. Researchers must be able to communicate their findings clearly and concisely to clinicians, patients, and the wider public. This involves using appropriate language, tailoring messages to different audiences, and employing various communication channels.
Translating complex scientific concepts into easily understandable information for diverse audiences is a critical aspect of successful communication and a critical component of bridging the gap between research and clinical practice.
Addressing the Economic Feasibility of Translation
The economic feasibility of translating research into clinical practice is a crucial factor. Developing new treatments and technologies often involves substantial financial investment in research, development, and clinical trials. Ensuring that these investments are justified and that the new treatments and technologies are cost-effective is vital to ensuring their successful implementation in healthcare systems.
Careful cost-benefit analyses are essential to assess the economic viability of translating research, ensuring that the investment in translation is aligned with the potential benefits for patients and the healthcare system.
Building Collaboration and Partnerships
Successful translation relies on strong collaboration and partnerships among researchers, clinicians, regulatory bodies, and industry stakeholders. Building effective partnerships facilitates the sharing of knowledge, resources, and expertise, accelerating the translation process. Collaboration also ensures that the needs and perspectives of different stakeholders are considered throughout the translation process, leading to more effective and impactful outcomes.
The Impact of Translational Research on Patient Outcomes

The Foundation of Translational Research
Translational research bridges the gap between basic scientific discoveries and their application in clinical practice. It's a crucial process for transforming laboratory findings into tangible benefits for patients. This process requires a meticulous and collaborative approach, involving researchers from various disciplines, from biologists and chemists to clinicians and engineers. Understanding the complexities of human health and disease is paramount in this process.
Identifying Promising Leads
A critical initial step in translational research is identifying promising leads from basic research. This involves rigorous analysis of data from laboratory experiments, animal models, and computational simulations. Researchers scrutinize potential therapeutic targets and mechanisms of action, assessing their feasibility and potential clinical impact. This careful selection process ensures that only the most promising candidates move forward into more advanced stages of development.
Developing Novel Therapies
Once promising leads are identified, the focus shifts to developing novel therapies or interventions. This involves designing and conducting preclinical studies to evaluate the safety and efficacy of these new approaches. Animal models play a crucial role in this stage, providing an opportunity to assess the potential benefits and risks before human trials. Developing new therapies requires expertise across various fields, including drug design, formulation, and delivery.
Clinical Trials and Validation
Clinical trials are the cornerstone of translational research, rigorously testing the efficacy and safety of new therapies in human subjects. These trials meticulously assess the treatment's effects on various parameters, from physiological responses to patient outcomes. The results of these trials are essential for validating the clinical utility of the interventions. Different phases of clinical trials assess various aspects, from initial safety to long-term efficacy and patient compliance.
Translating Research into Practice
Successful translational research culminates in the translation of findings into clinical practice. This involves developing guidelines, protocols, and educational materials for clinicians to effectively integrate the new therapies or interventions into routine care. This process ensures that patients benefit from the latest advancements in medicine. This also involves careful consideration of the ethical and societal implications of the findings.
Addressing Challenges and Future Directions
Despite significant progress, translational research faces numerous challenges. One major obstacle is the significant time and resources required for moving from laboratory discoveries to clinical application. Another crucial challenge lies in the coordination between researchers from different disciplines, requiring effective communication and collaboration. Future research should focus on improving the efficiency and effectiveness of the translational process to accelerate the development of new therapies and interventions, and to better address the needs of diverse patient populations.