Harnessing CRISPR for Enhanced Immune Responses
Harnessing CRISPR for Enhanced Immune Responses
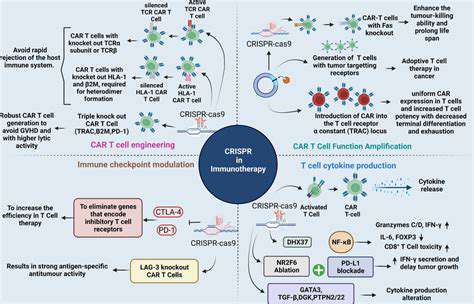
CRISPR-Cas9 gene editing technology has emerged as a revolutionary tool with immense potential to manipulate the human immune system. Its precise targeting capabilities allow scientists to modify immune cells, such as T cells, to enhance their ability to recognize and destroy cancerous cells or infected cells. This precise editing offers a pathway to potentially create more effective immunotherapies.
By directly modifying the genes responsible for immune cell function, researchers can potentially enhance the specificity and potency of these cells in combating disease. This precision editing could lead to more targeted therapies with reduced side effects compared to traditional treatments. The ability to tailor immune responses to specific pathogens or cancers holds tremendous promise for the future of medicine.
Improving Cancer Immunotherapy Efficacy
One of the most promising applications of CRISPR in immunology is its potential to improve cancer immunotherapy. CRISPR can be used to engineer T cells to recognize and attack cancer cells with greater efficiency. This involves modifying the T cells to express receptors that specifically bind to tumor-associated antigens, effectively turning them into highly targeted cancer-killing cells. This targeted approach has the potential to significantly reduce the off-target effects of current immunotherapies, minimizing harm to healthy tissues.
Scientists are exploring various strategies to enhance the efficacy of cancer immunotherapy using CRISPR. This includes enhancing the persistence and proliferation of engineered T cells within the tumor microenvironment. Such enhancements are crucial for achieving long-term remission and preventing tumor recurrence. Improved targeting and reduced toxicity are key to success in this area.
Addressing Limitations and Ethical Considerations
While the potential of CRISPR in enhancing immune responses is significant, there are also limitations and ethical considerations to address. One major concern is the potential for off-target effects, where the CRISPR system inadvertently modifies genes other than the intended target. Rigorous testing and validation are essential to minimize these risks and ensure the safety of these therapies. Careful monitoring and evaluation of the long-term effects of CRISPR-modified immune cells are crucial.
Ethical concerns regarding the use of CRISPR in human therapies must also be addressed. The potential for unintended consequences and the implications for germline editing raise important questions about the responsible use of this powerful technology. Open dialogue and regulatory frameworks are necessary to navigate these complexities ethically and ensure the responsible development and application of CRISPR in enhancing immune responses.
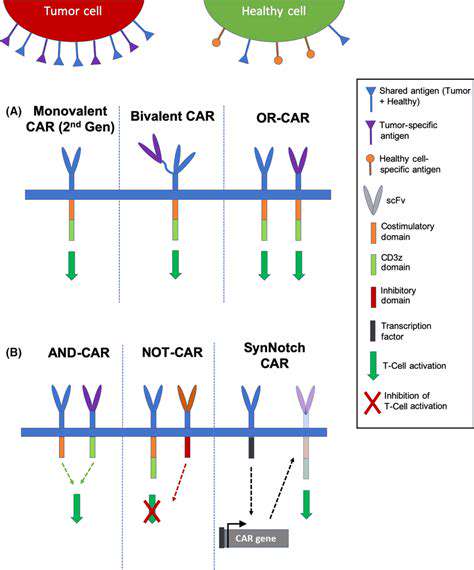
Tailoring T Cell Responses for Cancer Immunotherapy
Harnessing CRISPR-Cas9 for Targeted T Cell Engineering
CRISPR-Cas9 gene editing technology offers unprecedented precision in modifying T cells, enabling researchers to precisely target and manipulate specific genes within these crucial immune cells. This targeted approach allows for the introduction of novel functionalities, such as enhanced tumor recognition or resistance to immune checkpoints, directly into the T cell genome. The potential of this technology to engineer T cells with superior anti-cancer properties is a major driving force in the field of cancer immunotherapy.
The ability to precisely modify the genome of T cells with CRISPR-Cas9 opens up exciting possibilities for developing personalized cancer immunotherapies. Scientists can tailor T cells to recognize specific tumor antigens unique to individual patients, improving the efficacy and safety of treatment. This precision approach is a significant advancement over traditional therapies, which often struggle to target tumors effectively.
Engineering T Cell Receptors for Enhanced Tumor Recognition
Modifying T cell receptors (TCRs) is a crucial aspect of cancer immunotherapy. By engineering TCRs to recognize specific tumor-associated antigens, researchers can improve the ability of T cells to identify and eliminate cancer cells. This process involves identifying and utilizing unique tumor-specific epitopes, effectively creating a highly targeted immune response.
Advanced techniques allow for the design and selection of TCRs with enhanced specificity and affinity for tumor antigens. This leads to more potent anti-tumor responses and ultimately, improved treatment outcomes for patients.
Overcoming Immune Checkpoint Blockades with Gene Editing
Immune checkpoints, such as PD-1 and CTLA-4, play a crucial role in regulating the immune response. Cancer cells frequently utilize these checkpoints to evade detection and destruction by T cells. Gene editing can be employed to eliminate these inhibitory pathways, boosting the activity of T cells and enhancing their ability to attack tumors.
By directly targeting and disabling these checkpoints, gene editing strategies can create T cells that are less susceptible to tumor-induced immune suppression. This approach is particularly promising for overcoming resistance mechanisms employed by certain cancers.
Developing T Cell Persistence and Memory for Long-Term Efficacy
Ensuring long-term anti-tumor activity is essential for successful cancer immunotherapy. Gene editing can be used to enhance the persistence and memory of T cells, promoting a sustained immune response that can effectively combat the recurrence of cancer.
This approach involves introducing genes that promote T cell survival, proliferation, and memory formation. The goal is to create a long-lasting immune response that not only eradicates existing tumors but also prevents their recurrence.
Improving T Cell Trafficking and Infiltration into Tumors
Efficient infiltration of T cells into tumors is critical for effective anti-tumor activity. Gene editing can be used to modify T cells to improve their ability to migrate to and infiltrate tumor sites, enhancing their ability to directly engage and eliminate cancer cells.
This involves altering genes responsible for cell adhesion, chemotaxis, and other processes involved in immune cell trafficking. Improving T cell infiltration directly increases the likelihood of successful tumor eradication.
Addressing the Challenges of Off-Target Effects
Gene editing technologies, while powerful, are not without potential risks. Off-target effects, where the gene editing process unintentionally alters unintended genomic regions, are a crucial concern that must be addressed. Rigorous validation and optimization protocols are essential to minimize these risks and ensure the safety of gene-edited T cell therapies.
Ongoing research focuses on developing strategies to improve the precision and predictability of gene editing, significantly reducing the risk of off-target effects and enhancing the safety profile of these therapies. This is a key focus for ensuring that gene-edited T cells are safe and effective for clinical use.
Ethical Considerations and Regulatory Hurdles
The use of gene editing technologies in cancer immunotherapy raises important ethical considerations regarding the safety and long-term effects of these therapies. Thorough ethical review and stringent regulatory oversight are necessary to ensure responsible and appropriate use of these powerful tools.
The development of clear guidelines and regulations for the design, testing, and approval of gene-edited therapies is crucial for advancing the field while safeguarding patient safety and ethical considerations. This is a crucial step in ensuring the safe and responsible implementation of these innovative therapies.