The Growing Potential of Drug Repurposing for Rare Diseases
Identifying Suitable Candidates for Repurposing
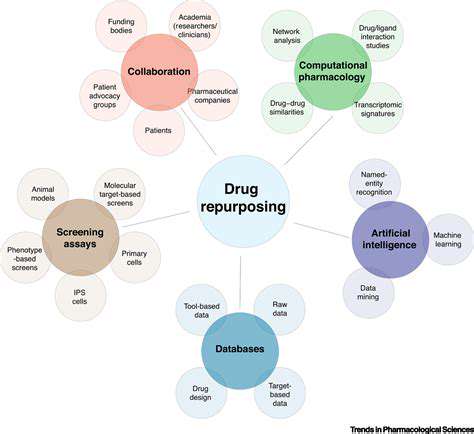
Drug repurposing presents a promising avenue for treating rare diseases, but meticulous screening is crucial to identify potential candidates. This involves evaluating existing drugs with known safety profiles and mechanisms of action that might be relevant to the pathophysiology of the rare disease. A deep dive into clinical trial data, preclinical studies, and existing literature is essential to understand the drug's efficacy and potential side effects in the context of the specific rare disease being considered. Careful consideration of the drug's dosage, administration route, and potential interactions with other medications is also paramount in this initial screening process.
The process isn't just about finding a drug that *might* work; it's about finding a drug that *is likely* to work with a minimal risk of adverse effects. This process requires collaboration between pharmaceutical companies, researchers, and patient advocacy groups to ensure the most promising candidates are prioritized for further investigation.
The Role of Genomics in Drug Repurposing
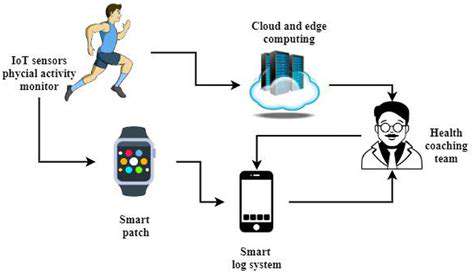
Genomic data plays a vital role in identifying potential drug targets and understanding the specific genetic mutations or pathways implicated in rare diseases. By analyzing the genetic makeup of patients with a particular rare disease, researchers can gain insights into the underlying molecular mechanisms driving the disease. This knowledge allows for a more targeted approach to drug repurposing, focusing on drugs that have demonstrated activity against the identified molecular pathways.
Addressing Challenges in Clinical Translation
Despite the potential benefits, translating repurposed drugs from the laboratory to clinical practice presents significant challenges. These challenges include ensuring the safety and efficacy of the drug in the specific patient population affected by the rare disease, often with limited sample sizes. Developing appropriate clinical trial designs and securing necessary funding are crucial steps in overcoming these obstacles. Regulatory hurdles can also delay the process, requiring rigorous evidence to demonstrate the drug's effectiveness and safety in treating the rare disease.
Ethical Considerations and Patient Engagement
Ethical considerations are paramount in drug repurposing research, particularly in the context of rare diseases. Ensuring informed consent from patients and their families is essential, acknowledging the potential risks and benefits of participation in research studies. The development of robust data-sharing protocols is also critical, acknowledging patient privacy and data security concerns. Active patient engagement throughout the research process is vital, ensuring their voices and needs are heard and incorporated into the development of appropriate treatments.
Funding and Resource Allocation for Rare Diseases
Adequate funding and resources are essential to support the research and development of repurposed drugs for rare diseases. Public-private partnerships, philanthropic organizations, and government initiatives can play a significant role in providing the necessary financial support. Prioritizing research into rare diseases through dedicated funding mechanisms can accelerate the discovery and development of effective treatments. This includes providing resources for clinical trials, data analysis, and the overall process of bringing repurposed drugs to market.
Potential Benefits and Limitations of Drug Repurposing
Drug repurposing offers a potentially faster and more cost-effective path to developing treatments for rare diseases compared to traditional drug development. The availability of pre-existing safety data and knowledge of the drug's mechanism of action can significantly reduce the time and resources required. However, limitations exist. Repurposed drugs may not be optimally suited for the specific needs of rare diseases. Furthermore, the existing dosage and administration protocols may require adjustments for optimal efficacy and safety in these patient populations. Careful consideration of these factors is crucial for successful repurposing initiatives.
The Future of Drug Repurposing in Rare Disease Treatment
The Growing Need for Novel Therapeutics
The pharmaceutical industry faces a significant challenge in developing new drugs to combat various diseases. The development process is often lengthy, expensive, and fraught with uncertainties. Consequently, there's a growing emphasis on repurposing existing drugs, which have already undergone rigorous testing and safety evaluations. This approach can significantly accelerate the drug discovery process and potentially reduce costs.
Identifying Potential Drug Candidates
A crucial aspect of drug repurposing is identifying potential candidates. This involves analyzing existing drug databases, exploring clinical trial data, and utilizing bioinformatics tools to identify drugs with potential activity against specific targets related to the disease of interest. Careful consideration of the drug's mechanism of action and potential side effects is paramount in this process.
Leveraging Big Data and Artificial Intelligence
The volume of biomedical data is exploding, creating a fertile ground for the application of big data analytics and artificial intelligence. These technologies can assist in identifying potential drug repurposing targets with significantly greater speed and accuracy than traditional methods. This accelerates the process of finding suitable drugs for specific diseases. Furthermore, AI can analyze complex patterns in data, potentially uncovering correlations that human researchers might miss.
Clinical Trial Design and Implementation
Once a potential drug candidate is identified, rigorous clinical trials are essential to evaluate its efficacy and safety in humans. These trials must be meticulously designed to ensure that data collected is reliable and unbiased. The trials should also focus on specific patient populations, potentially leading to more targeted treatments and improved outcomes. Data collected from these trials will also provide crucial insights into the drug's potential benefits and risks.
Ethical Considerations and Regulatory Hurdles
Drug repurposing, while promising, faces ethical considerations and regulatory hurdles. These factors must be carefully evaluated and addressed. Transparency and rigorous ethical review boards are essential to ensure that the process is conducted responsibly and with patient safety as a priority. Furthermore, regulatory bodies need to adapt to the specific requirements of repurposing, ensuring that new drugs meet the same high standards of safety and efficacy as those developed from scratch.
The Potential Impact on Healthcare
Drug repurposing has the potential to revolutionize healthcare by accelerating the development of new treatments for various diseases. This could lead to more affordable and accessible therapies, particularly for conditions that currently lack effective treatments. By repurposing existing drugs, we can potentially reduce the burden on healthcare systems and improve the lives of countless patients worldwide. It offers a powerful strategy for accelerating the development of novel therapies.