Introduction to Gene Editing in Immunotherapy
Understanding Gene Editing
Gene editing, a revolutionary field, involves altering an organism's DNA. This precise modification of genetic material holds immense potential for treating a wide range of diseases, including cancer. The ability to target and modify specific genes within cells opens doors to therapies that were previously unimaginable. Techniques like CRISPR-Cas9 are at the forefront of this exciting advancement, offering a powerful tool for researchers and clinicians.
Different gene editing methods exist, each with its own strengths and limitations. Understanding these nuances is crucial for developing effective and safe therapeutic strategies. Careful consideration of potential off-target effects and the long-term consequences of genetic alterations is essential for responsible application.
The Role of Immunotherapy in Cancer Treatment
Immunotherapy harnesses the body's own immune system to fight cancer. It has shown remarkable success in treating certain cancers, but limitations remain in effectively targeting and eliminating diverse tumor types. One of the key challenges is the ability of tumors to evade the immune system, rendering them less susceptible to treatment.
By combining gene editing with immunotherapy, researchers aim to enhance the immune response, making it more effective against cancer cells. This strategy involves modifying immune cells to recognize and destroy cancer cells with greater precision and efficiency.
Gene Editing Techniques in Immunotherapy
CRISPR-Cas9 is a powerful gene editing tool that has revolutionized the field. Its ability to precisely target and modify DNA sequences has opened doors to creating immune cells with enhanced anti-tumor activity. This technology allows researchers to modify T cells, a crucial component of the immune system, to recognize and attack cancer cells more effectively.
Other gene editing approaches, such as zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs), also play a role in immunotherapy research. These methods offer alternative strategies for modifying immune cells, each with its own set of advantages and disadvantages.
Enhancing Immune Cell Functionality
Gene editing can be used to enhance the functionality of immune cells, such as T cells and natural killer (NK) cells. This enhancement can involve improving their ability to recognize and kill cancer cells, increasing their persistence in the body, and reducing their tendency to be suppressed by the tumor microenvironment.
Modifying genes that regulate immune cell activation, cytokine production, and trafficking can lead to more potent anti-cancer responses. The goal is to create immune cells that are more effective at targeting and eliminating tumor cells in the body.
Improving Cancer Targeting and Specificity
Gene editing can be employed to improve the specificity of immune cells, ensuring that they target only cancer cells and avoid harming healthy tissue. This is crucial for minimizing side effects and maximizing the effectiveness of immunotherapy.
This includes modifying the immune cells' ability to recognize specific cancer antigens, ensuring they selectively destroy cancer cells without attacking normal cells. Precision targeting reduces the risk of damaging healthy tissues and improving the safety profile of the therapy.
Ethical Considerations and Future Directions
Gene editing in immunotherapy raises ethical considerations regarding the safety and potential risks associated with altering the human genome. Rigorous preclinical testing and careful clinical trials are essential to ensure responsible implementation of these novel therapies.
Future research will focus on developing more precise and efficient gene editing techniques, improving the safety profiles of these therapies, and expanding their application to a broader range of cancers. Continued research and development will be critical for realizing the full potential of gene editing in immunotherapy.
Enhancing T-Cell Function with Gene Editing
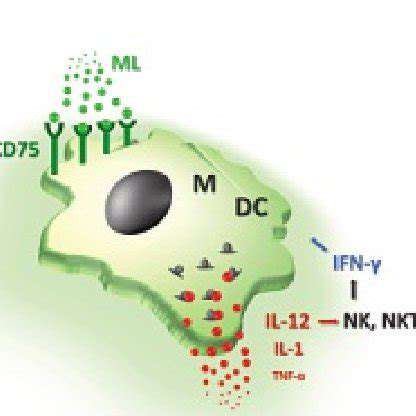
Gene Editing Strategies for Enhanced T-Cell Function
Gene editing technologies, particularly CRISPR-Cas9, offer a powerful approach to manipulate the genetic makeup of T cells, potentially enhancing their function and efficacy in immunotherapy. Precise targeting of genes involved in T-cell activation, proliferation, and cytokine production allows for the development of customized therapies tailored to individual patient needs. This precision is crucial for minimizing off-target effects and maximizing the desired therapeutic outcome. The ability to engineer T cells with enhanced anti-tumor activity is a significant step forward in cancer treatment.
Improving T-Cell Persistence and Activity
A critical aspect of successful immunotherapy is the ability of engineered T cells to persist in the body and maintain their anti-tumor activity over time. This can be achieved by introducing genes that promote T-cell survival, or by inhibiting programmed cell death pathways. Strategies for improving T-cell persistence are crucial for long-term efficacy and preventing tumor relapse. By modifying specific genes, researchers can potentially extend the lifespan of these crucial immune cells, enabling them to effectively eliminate cancerous cells for an extended period.
Targeted Cytokine Production for Enhanced Response
The ability of T cells to secrete specific cytokines, such as interferon-gamma and tumor necrosis factor-alpha, is essential for coordinating an effective immune response. Modifying the expression of genes related to cytokine production can potentially enhance the magnitude and duration of the immune response against tumors. Altering cytokine profiles may also improve the safety and reduce potential side effects associated with T-cell therapies. Careful consideration of the specific cytokine targets is essential to avoid unwanted immune responses.
Engineering T-Cell Receptors for Expanded Specificity
T-cell receptors (TCRs) are crucial for recognizing and targeting specific antigens. Engineering TCRs with expanded specificity can broaden the range of targets that T cells can recognize, including those that may be hidden from the immune system in a tumor microenvironment. This strategy can improve the efficacy of T-cell-based therapies in treating tumors that are resistant to standard approaches. Increasing the range of specificities through genetic modification of TCRs could potentially lead to improved outcomes in previously untreatable cancers.
Delivery Systems and Safety Considerations
Effective delivery of gene-edited T cells to the patient is a critical aspect of this approach. Developing safe and efficient methods for delivering these modified cells to the target tissues is crucial for clinical translation. Safety concerns regarding off-target effects and potential immune responses to the modified cells must be rigorously addressed in preclinical and clinical studies. Careful evaluation of the safety profile of each gene editing strategy is essential to ensure the safety of patients receiving these potentially life-altering therapies. The development of appropriate delivery systems and safety protocols is paramount.
Targeted Antigen Receptor Engineering for Enhanced Specificity
Targeted Antigen Receptor Engineering for Enhanced Specificity
Engineered antigen receptors (CARs) hold immense promise for cancer immunotherapy, enabling the reprogramming of T cells to recognize and eliminate tumor cells. However, achieving optimal specificity and minimizing off-target effects remains a critical challenge. Precise engineering of the antigen-binding domain is paramount for maximizing the therapeutic index of these engineered immune cells. This requires innovative strategies to target specific epitopes and minimize cross-reactivity with healthy tissues.
Precise Epitope Targeting
A key aspect of enhancing specificity involves precise targeting of specific epitopes. Instead of targeting broadly expressed proteins, researchers are focusing on identifying unique, tumor-specific epitopes that are not present on normal cells. This meticulous approach ensures that the engineered T cells recognize and destroy only the target cancer cells while sparing healthy tissue, thereby reducing adverse effects.
Identifying and validating these unique epitopes requires sophisticated bioinformatics tools and experimental validation. This process often involves extensive screening and characterization to ensure the selected epitope is truly tumor-specific and not expressed in healthy cells.
Minimizing Off-Target Binding
Minimizing off-target binding is another crucial aspect of improving specificity. This involves carefully selecting and modifying the antigen-binding domain of the CAR to reduce its affinity for non-target antigens. Computational modeling and extensive in vitro assays are employed to evaluate the binding affinity of the engineered receptor for various antigens, both target and non-target.
Rational Design of Antigen Recognition Motifs
Rational design approaches are being employed to engineer novel antigen recognition motifs. This involves considering the structural characteristics of the target antigen and designing CARs that exhibit optimal binding affinity and selectivity. Advanced biophysical techniques, such as surface plasmon resonance and isothermal titration calorimetry, are essential to optimize these engineered receptors.
Utilizing CRISPR/Cas9 for Precise Modifications
Gene editing technologies, like CRISPR/Cas9, are providing unprecedented opportunities for precise modifications to the antigen receptor. Using this technology, researchers can introduce precise mutations into the antigen-binding domain, fine-tuning the receptor's specificity and affinity for the target antigen. This approach allows for targeted modification of existing CARs or the development of entirely novel receptor designs.
Validation and Preclinical Evaluation
Thorough validation is essential to ensure the safety and efficacy of engineered antigen receptors. Rigorous preclinical studies are required to assess the specificity and potency of the engineered immune cells in various tumor models. These studies should evaluate the ability of the engineered T cells to effectively eliminate target cells while minimizing off-target effects and toxicity. Safety and tolerability are crucial to the success of these therapies in human clinical trials.
Challenges and Future Directions in Gene Editing Immunotherapy
Overcoming Existing Limitations
One of the primary challenges in the field of artificial intelligence is the inherent limitations of current algorithms. While impressive feats are being accomplished, many AI systems struggle with nuanced understanding, common sense reasoning, and adapting to unforeseen circumstances. These limitations often manifest as a lack of generalizability, hindering the ability of AI to effectively perform tasks outside the specific training data they have been exposed to. This underscores the need for continued research and development to overcome these barriers, paving the way for more robust and versatile AI systems.
Furthermore, the computational resources required for training complex AI models can be substantial, posing a significant barrier to accessibility and widespread adoption. Efficient algorithms and hardware advancements are crucial to address this hurdle and democratize the use of AI technologies across various sectors.
Addressing Ethical Concerns
The rapid advancement of AI brings forth a multitude of ethical concerns that require careful consideration. Bias in algorithms, stemming from the data used for training, can perpetuate and even amplify existing societal inequalities. Addressing these biases is paramount to ensuring fairness and equity in AI applications. Developing methods for detecting and mitigating bias is a critical area of research.
Another critical ethical concern surrounds the potential for misuse of AI technologies. Ensuring responsible development and deployment of AI systems is essential to prevent unintended harm and protect vulnerable populations. Robust regulations and ethical guidelines are needed to navigate these challenges.
Enhancing Explainability and Transparency
Many current AI systems, especially deep learning models, operate as black boxes, making it difficult to understand how they arrive at their conclusions. This lack of explainability hinders trust and acceptance in AI systems, especially in critical applications like healthcare and finance. Developing methods for explaining AI decisions is crucial for building public confidence and fostering responsible use. This includes techniques for visualizing internal workings and providing clear justifications for predictions.
Greater transparency in the development and deployment processes of AI systems is also needed. Clear documentation, open-source models, and standardized evaluation metrics can enhance trust and facilitate scrutiny. This openness is essential for the development of a robust and trustworthy AI ecosystem.
Exploring New Applications and Domains
The field of AI has the potential to revolutionize countless sectors, from healthcare and education to transportation and manufacturing. Exploring new applications in under-served areas is critical to ensuring that the benefits of AI are accessible to all. This includes developing solutions for personalized medicine, educational support, and improved transportation systems.
AI has the potential to address global challenges like climate change, poverty, and disease. Continued research into innovative solutions in these areas can accelerate progress towards a more sustainable and equitable future. For example, AI can analyze vast amounts of data to identify patterns and predict future trends in climate change or optimize resource allocation in poverty-stricken regions.
Fostering Collaboration and Knowledge Sharing
The advancement of AI requires collaboration across disciplines, from computer science and engineering to social sciences and humanities. Bridging these gaps through interdisciplinary research and knowledge sharing is essential to address the multifaceted challenges in developing responsible and beneficial AI systems. Collaboration can accelerate innovation and foster a more holistic approach to AI development.
Open-source platforms, shared datasets, and collaborative research projects are crucial for fostering a shared understanding and driving progress in the field. Promoting public-private partnerships and fostering international cooperation can accelerate the development of impactful AI solutions.