CRISPR's Precision Challenges
Despite its revolutionary potential, CRISPR-Cas9, the most widely used gene-editing tool, isn't without its limitations. One significant hurdle is the inherent off-target effects. While CRISPR aims to modify specific DNA sequences, it can sometimes inadvertently alter other, unrelated parts of the genome. This off-target activity can lead to unintended consequences, potentially causing harmful mutations and raising concerns about the safety of the technology, especially in therapeutic applications. Researchers are constantly working to refine CRISPR techniques and design guide RNAs to minimize these off-target effects, but the challenge remains significant.
Another limitation lies in the delivery of the CRISPR components into target cells. Efficient delivery systems are crucial for in vivo gene editing applications. Delivering the Cas9 enzyme and guide RNA to the precise cells and tissues in the body is a complex problem. Currently available methods often struggle with efficiency and specificity, leading to low editing rates and potential toxicity to healthy cells. Developing more efficient and targeted delivery systems is a critical area of ongoing research, crucial for the widespread adoption of CRISPR in therapeutic settings.
Beyond CRISPR: Exploring Alternative Gene Editing Methods
The limitations of CRISPR have spurred research into alternative gene editing technologies. These methods are still in various stages of development, but they offer potential advantages in terms of precision, efficiency, and safety. One such approach is base editing, which directly alters individual DNA bases without the need to introduce a double-strand break. This method holds promise for treating genetic diseases caused by single-nucleotide polymorphisms, offering a more precise and potentially safer alternative to CRISPR.
Another promising avenue is prime editing, which allows for more complex genetic alterations than base editing. It can introduce insertions, deletions, or even more intricate modifications to the DNA sequence. Prime editing's ability to precisely target and modify specific DNA sequences with minimal off-target effects makes it an attractive alternative to CRISPR, particularly for correcting more complex genetic defects.
Further developments include transcription activator-like effector nucleases (TALENs) and zinc finger nucleases (ZFNs), which are older gene editing technologies. While CRISPR has largely superseded these methods in popularity, some researchers continue to explore and refine them for specific applications where CRISPR might not be the ideal solution. These alternatives are still under investigation and hold promise for specific applications, but their widespread use is less frequent due to CRISPR's current dominance.
The pursuit of these alternative gene editing techniques is crucial for enhancing the precision, safety, and efficiency of genome modification, ultimately paving the way for more effective and less risky therapeutic interventions.
These alternative methods, while still in the research phase, demonstrate the ongoing quest to find more versatile and precise tools for gene editing. The field is dynamic, with researchers continually developing new approaches to overcome the limitations of existing technologies.
Base Editing: A Precise Approach to DNA Modification
Base Editing: A Revolutionary Gene-Editing Technique
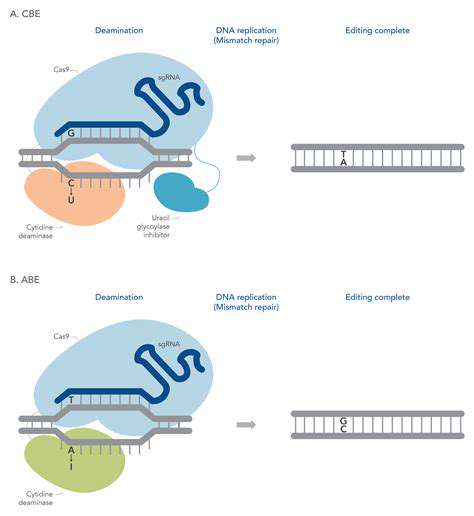
Base editing is a revolutionary gene-editing technology that offers a more precise and efficient approach compared to previous methods like CRISPR-Cas9. This novel technique allows scientists to directly modify single DNA bases within a target gene sequence, making it a powerful tool for treating genetic diseases. This precision minimizes the potential for off-target effects, a significant concern in other gene-editing methods. Base editing is potentially less disruptive to the genome than other gene-editing methods, offering a more tailored approach to correcting genetic defects.
Unlike CRISPR-Cas9, which typically introduces double-strand breaks in the DNA, base editing employs engineered enzymes that directly convert one DNA base to another. This targeted alteration, without the need for a complete DNA repair mechanism, leads to a more predictable and controlled outcome. This targeted approach significantly reduces the chances of unwanted mutations in other parts of the genome, a crucial aspect for therapeutic applications.
Mechanism of Action: How Base Editing Works
Base editing relies on a fusion of a DNA base editor with a DNA targeting component, often derived from CRISPR-Cas systems. These enzymes are engineered to recognize and bind to the specific DNA sequence of the target gene. This targeted binding is critical for precision, ensuring that the editing occurs only at the intended location. The base editor then catalyzes a chemical reaction that converts one DNA base to another, effectively altering the gene's sequence.
The process typically involves two key components: a DNA targeting system (often CRISPR-Cas) and a specialized enzyme called a base editor. The targeting system guides the base editor to the precise location within the genome. The base editor then performs the chemical conversion of the specific target base, ensuring that the change occurs at the desired position in the DNA sequence. This targeted approach minimizes the risk of unwanted alterations in other parts of the genome, which is a significant safety advantage compared to earlier gene-editing technologies.
Applications and Potential Benefits
Base editing holds immense potential for treating a wide range of genetic diseases. Its ability to precisely modify single DNA bases opens doors for correcting mutations responsible for conditions like sickle cell anemia, cystic fibrosis, and Huntington's disease. This precision also makes it a compelling tool for research, enabling scientists to study the function of specific genes and their role in various biological processes. Targeted gene correction without the risks of double-strand breaks is a significant advantage over other gene-editing techniques.
Beyond disease treatment, base editing has applications in agriculture. Scientists are exploring its potential to enhance crop yields and nutritional value by modifying genes responsible for traits like drought tolerance and nutrient content. This precision approach also holds the potential for advancing fundamental biological research by enabling the targeted alteration of genes in various organisms, helping scientists better understand gene function and cellular processes.
Challenges and Future Directions
While base editing shows great promise, challenges remain. One significant hurdle is optimizing the efficiency and specificity of base editing enzymes. Scientists are working to develop more effective and precise base editors that can target a wider range of DNA sequences and minimize off-target effects. Further research is needed to fully assess the long-term effects of base editing on the genome and potential cellular responses to these modifications.
Another key area of focus is expanding the types of DNA base modifications that can be achieved with base editing. Scientists are investigating the possibility of extending the technique to more complex genetic alterations or to incorporate additional functionalities into the base editor. These advancements could pave the way for even more sophisticated therapeutic interventions and biological research. Continued research and development are crucial for addressing these challenges and fully realizing the transformative potential of base editing.
Prime Editing: Writing and Rewriting the Genetic Code

Prime Editing: A Revolutionary Gene Editing Technique
Prime editing, a revolutionary gene-editing technology, offers a powerful new approach to correcting genetic defects. Unlike previous methods, it allows for precise and targeted rewriting of DNA sequences, rather than simply making cuts and relying on cellular repair mechanisms. This capability holds immense potential for treating a wide range of genetic diseases, offering a significant advancement in the field of genetic medicine.
This technology is a significant advancement over previous gene editing techniques like CRISPR-Cas9, as it allows for more precise and targeted changes to the DNA sequence. It overcomes some of the limitations of CRISPR-Cas9 by potentially reducing off-target effects and improving the accuracy of gene editing.
Mechanism of Action
Prime editing utilizes a unique protein complex, composed of a prime editor enzyme and a guide RNA. The guide RNA directs the prime editor to the precise location in the genome needing modification. The prime editor then directly rewrites the DNA sequence, without the need for the cell's error-prone repair mechanisms. This direct rewriting capability is a key advantage over previous methods, as it reduces the risk of unintended mutations.
Prime editing's mechanism of action involves a process where the prime editor enzyme makes a temporary single-stranded break in the target DNA. This break allows for the introduction of a desired sequence, which is then incorporated into the DNA strand. The accuracy of this incorporation process is significantly higher compared to other gene editing approaches.
Applications and Potential Benefits
The potential applications of prime editing are vast and span a range of genetic diseases. It holds promise for treating inherited disorders, such as cystic fibrosis, sickle cell anemia, and Huntington's disease, by precisely correcting the faulty genes responsible for these conditions. The ability to directly rewrite the DNA sequence without relying on error-prone cellular repair mechanisms could lead to a significant reduction in unwanted side effects.
This technology also has the potential for advancements in agriculture. Precise gene editing could lead to crops with enhanced traits, such as increased yield or resistance to pests and diseases. Furthermore, prime editing may pave the way for the development of novel therapies for cancers and other complex diseases.
Challenges and Future Directions
While prime editing holds immense promise, several challenges need to be addressed before it can be widely implemented in clinical settings. These include optimizing the efficiency and specificity of the prime editor enzyme, further characterizing potential off-target effects, and ensuring long-term safety and efficacy in vivo.
Ongoing research is focused on overcoming these challenges and exploring the full potential of prime editing. Researchers are also working on developing improved delivery systems for the prime editor complex, which will be crucial for effective application in different tissues and organs. The future of prime editing looks promising, with the potential to revolutionize gene therapy and improve human health.
Transcription Activator-Like Effector Nucleases (TALENs) and Zinc Finger Nucleases (ZFNs): Pioneers in the Field
Transcription Factor Function
Transcription activator-like effectors (TALEs) are a class of DNA-binding proteins that play a crucial role in regulating gene expression in various organisms, particularly plants. These proteins are characterized by their ability to specifically bind to DNA sequences, thereby influencing whether a gene is turned on or off. TALEs achieve this precise targeting through a highly modular structure, allowing for the design and engineering of customized DNA-binding domains.
Understanding the mechanisms behind TALE function is essential for manipulating gene expression for various applications, such as improving crop yields or developing disease-resistant plants. The modular nature of TALEs makes them attractive tools for targeted gene editing, offering a level of precision not readily available with other methods.
Molecular Structure and Mechanism
The remarkable versatility of TALEs stems from their unique structure. They consist of repeating 33-35 amino acid motifs, each responsible for recognizing a specific base pair in the DNA sequence. This modular design allows for the precise engineering of TALE proteins to target any desired DNA sequence.
The mechanism of TALE-DNA interaction is crucial for understanding their function. Each repeat motif interacts with a specific base pair in the DNA helix. This interaction is highly specific, allowing TALEs to bind to their target DNA sequence with high affinity and specificity.
Applications in Gene Editing
The modular nature of TALEs makes them valuable tools in gene editing. Researchers can engineer TALEs to bind to specific DNA sequences, enabling them to precisely target and modify genes of interest. This precision is a significant advantage over other gene editing methods, potentially minimizing off-target effects.
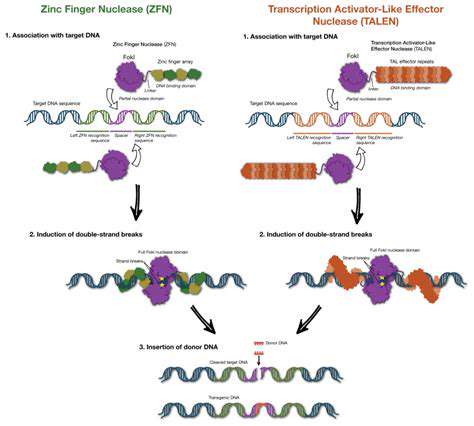
TALENs (Transcription Activator-Like Effector Nucleases), a derivative of TALEs, incorporate a nuclease domain into the protein. This allows for the precise cutting of DNA at the target site, opening up possibilities for gene disruption or targeted gene replacement. This is a powerful technique with significant implications for agricultural biotechnology and biomedical research.
Comparison with Other Gene Editing Tools
TALEs offer a compelling alternative to other gene editing tools, such as zinc finger nucleases (ZFNs). While ZFNs also allow for targeted gene editing, the design and engineering of ZFNs can be more complex and time-consuming. The modular nature of TALEs simplifies the design process, making them more accessible and efficient for researchers.
The development of CRISPR-Cas9, though more recent, has in some instances superseded TALEs in popularity due to its simplicity and ease of use. However, TALEs still hold significant advantages in certain contexts, particularly where precise control over the binding site is paramount.
Future Directions and Research
Ongoing research continues to explore the potential of TALEs in various applications. Scientists are investigating their use in developing novel therapies for genetic diseases, such as cystic fibrosis and Huntington's disease. Further research into the mechanisms of TALE-DNA interaction and the optimization of TALE design could lead to even more sophisticated applications.
Improved understanding of the factors influencing TALE activity and specificity will be crucial for enhancing their efficiency and minimizing potential risks associated with off-target effects. This research promises to expand the horizons of gene editing and therapeutic interventions.